About STARS
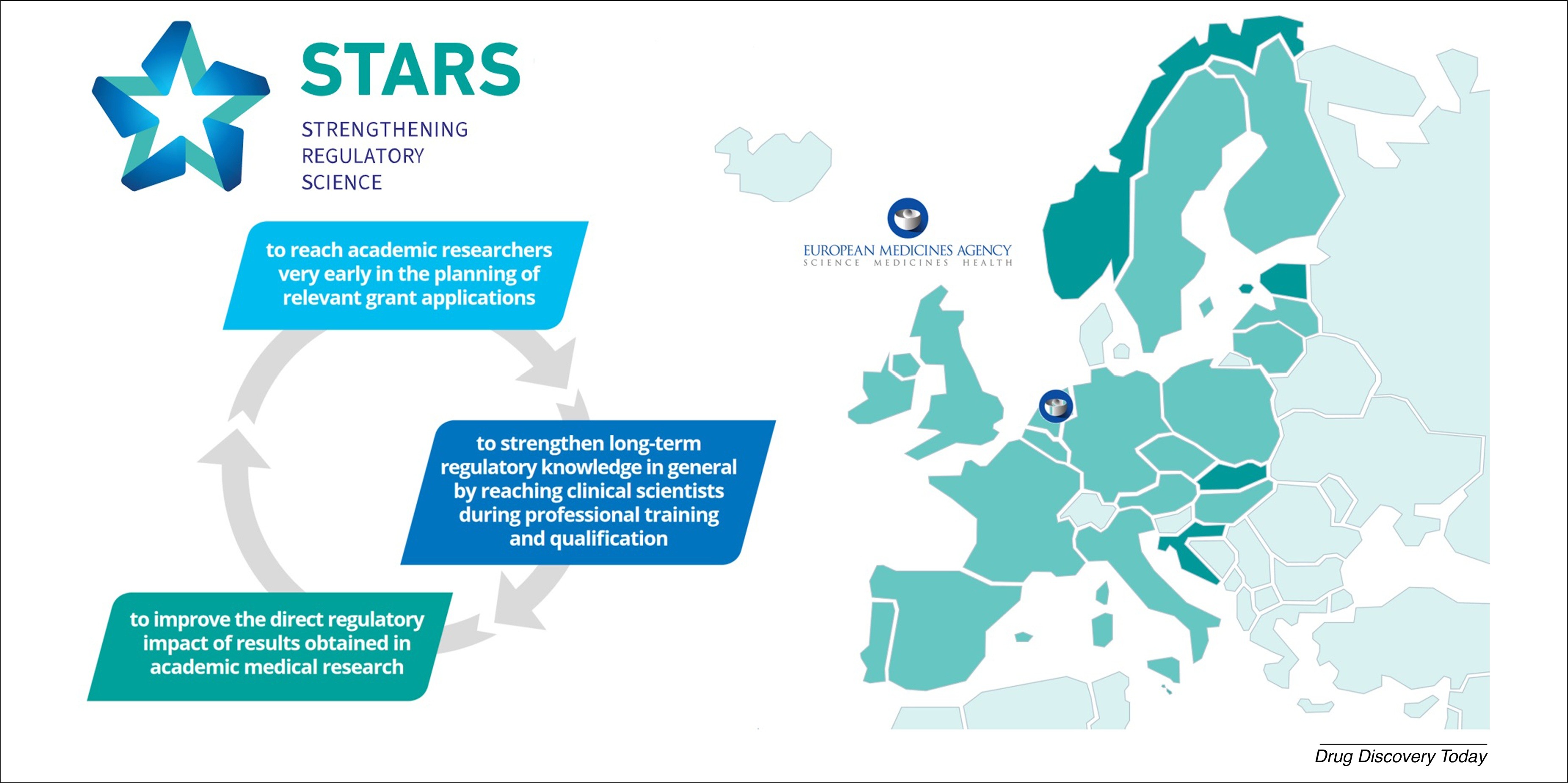
The EU-funded project Strengthening Training of Academia in Regulatory Sciences (STARS) aimed at improving regulatory education and support activities to enhance success and outcome during regulatory scientific advice procedures. The project consequently addressed three major aims:
- improving general knowledge on regulatory issues in the academic world,
- improving the dialogue and communication between relevant stakeholders, and
- strengthening the support for successful outcomes during regulatory scientific advice with direct regulatory impact of academic driven research.
STARS was a Coordination and Support Action (CSA) and was a collaboration between 19 European national competent authorities (NCAs) from 18 countries, four associate countries, the European Medicines Agency (EMA) and the DLR Project Management Agency. The project was supported by the European Commission’s (EC) Framework Program for Research and Innovation Horizon 2020 under grant agreement number 825881. It was concluded on 30 June 2022.
On the following pages, you will find the key outcomes of the project, including the STARS curricula, important training materials, and publications. In-depth and detailed descriptions of the individual work packages and results can be found in the STARS Common Strategy. Please note that all content and materials presented here have not been updated since the end of the project (June 2022).
Project Coordination
- Federal Institute for Drugs and Medical Devices (BfArM), Germany
- Paul-Ehrlich Institute (PEI), Germany
- DLR Projektträger, Germany
Project partners (in alphabetical order)
- Agency of the Dutch Medicines Evaluation Board (CBG / MEB), The Netherlands
- Austrian Agency for Health and Food Safety (AGES), Austria
- Czech State Institute for Drug Control (SÚKL), Czech Republic
- European Medicines Agency (EMA), EU
- Federal Agency For Medicines And Health Products (FAMHP), Belgium
- Finnish Medicines Agency (FIMEA) , Finnland
- French Agency for the Safety of Medicines and Health Products (ANSM), France
- Health Products Regulatory Authority (HPRA), Ireland
- Italian Medicines Agency (AIFA), Italy
- Medical Products Agency (MPA), Sweden
- Medicines And Healthcare Products Regulatory Agency (MHRA), UK
- Medicines Authority (MA), Malta
- National Authority of Medicines and Health Products (INFARMED), Portugal
- National Institute of Pharmacy and Nutrition (OGYÉI), Hunagry
- Office for Registration of Medicinal Products, Medical Devices and Biocidal Products (URPL), Poland
- Spanish Agency of Medicines and Medical Devices (AEMPS), Spain
- State Agency of Medicines of Latvia (ZVA / SAMLV), Latvia
- State Medicines Control Agency (VVKT), Lithuania
Associated project partners (in alphabetical order)
- Agency for Medicinal Products and Medical Devices (HALMED), Croatia
- Norwegian Medicines Authority (NOMA), Norway
- State Agency of Medicines (SAM), Estonia
Contact
Email: coordinationSTARS@bfarm.de
