How many medicinal products are marketable in Germany? How many clinical trials are registered each year? How many risk reports for medical devices are received by the BfArM? This chapter presents data and statistics that exemplify the work and diversity of Europe's largest authority in the field of licensing and safety of medicinal products and medical devices.
BfArM as employer
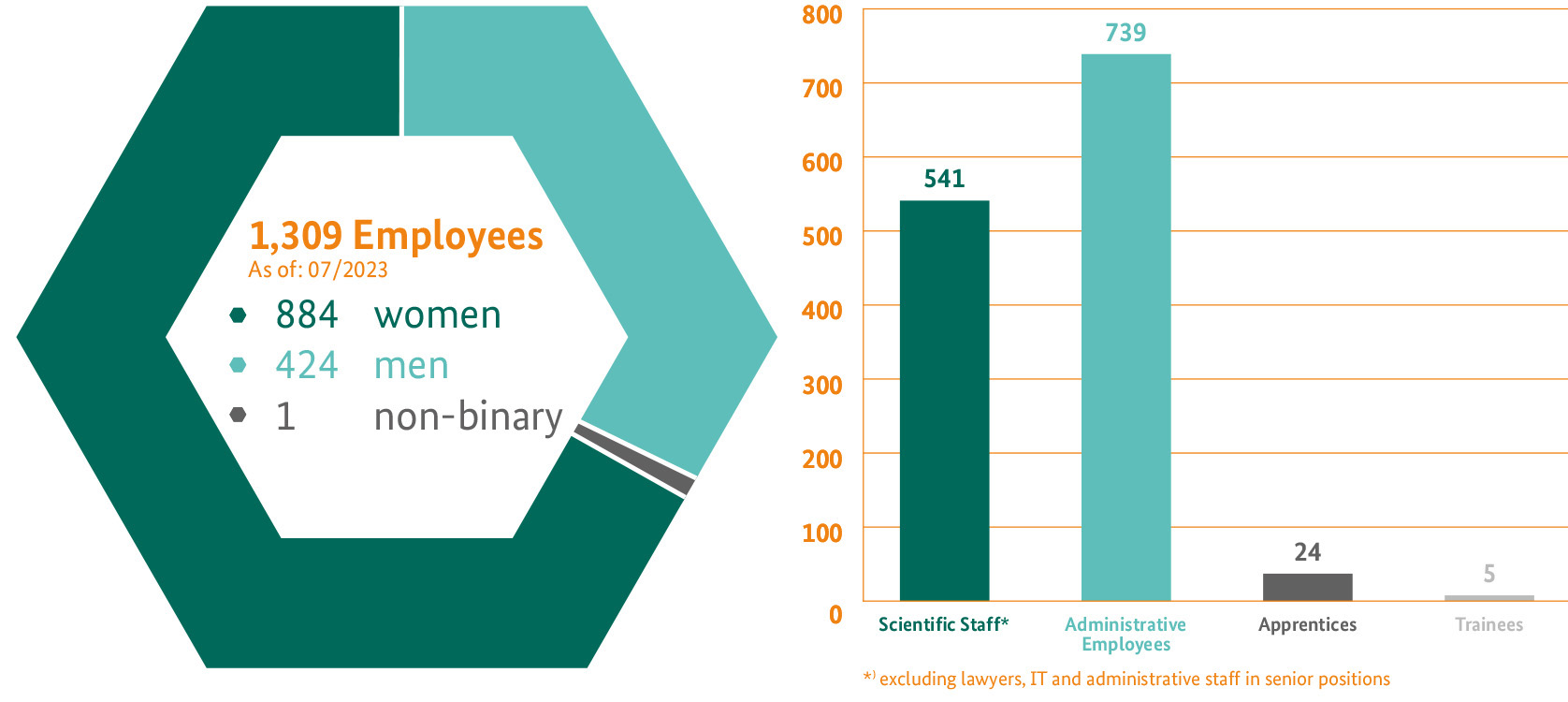
The BfArM is the largest European body in the area of licensing and safety of drugs and medical devices. Around 1,300 employees in a wide range of disciplines are committed to the safe care of patients.
Of the scientific staff members, around 31 percent are pharmacists; around 22 percent are medics; almost half (around 47 percent) are made up from scientific professional categories.
109
applications for acceptance into the DiGA register
... received by the BfArM in 2021 and 2022. 32 applications were accepted after testing.
210
consultations
... on digital healthcare applications (DiGA) performed by the BfArM in 2021 and 2022.
290
national scientific consulting processes
... performed by the BfArM in 2022. This could include: advice in the development phase, before requesting a clinical trial or before applying for a license..
33
total number of kick-off meetings
... performed by the BfArM Innovation Office in 2021 and 2022. Academic research groups, small and medium-sized enterprises as well as start-ups have the option here to receive guidance on early medicines and medical device development.
Drug approval
Decentralised licensing processes: leading procedures BfArM
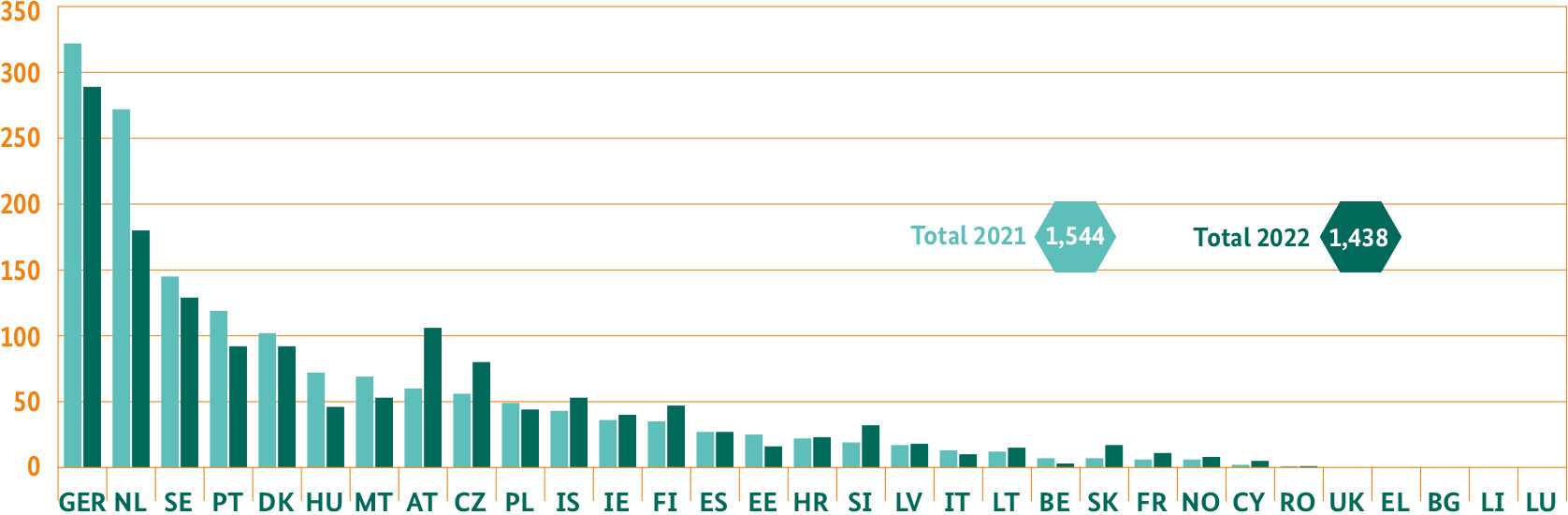
The BfArM has confirrmed its leading position over recent years in the European network of all licensing bodies for medicinal products. During the Covid-19 pandemic, the BfArM fully continued its processing of procedures as a Reference Member State (RMS) despite many additional tasks. The RMS is responsible for lead processing. In 2021, the BfArM looked after 21 percent of all EU procedures carried out decentrally as an RMS. This includes the DCP (Decentralised Procedures) and Mutual Recognition Procedures (MRP). In 2022, as an RMS, the BfArM participated in 20 percent of all EU procedures carried out decentrally.
Central licensing procedures via the European Medicines Agency (EMA)
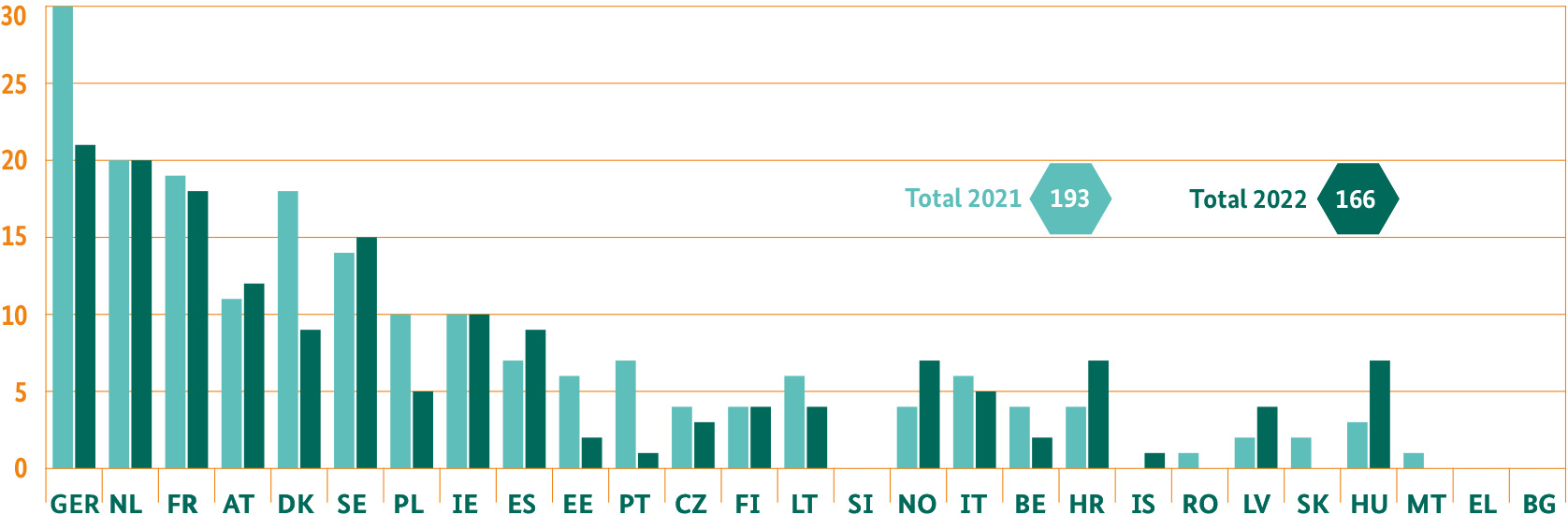
The BfArM also takes on a leading role in the centralised licensing processes for innovative medicines. Licensing of the appropriate medicines for these procedures automatically applies for the whole of the European Economic Area – unlike for the decentralised procedure. In 2021, Germany provided the rapporteur or co-rapporteur for 30 centralised procedures, which is the reporting or leading authority (The BfArM and Paul-Ehrlich Institut, PEI). In both 2021 and 2022, the BfArM was lead manager for 15 procedures. In 2021, it also supported the PEI, which was responsible for vaccines, in individual procedures with the assessment of the vaccine quality.
German Clinical Trials Register (DRKS)
Registrations performed each year
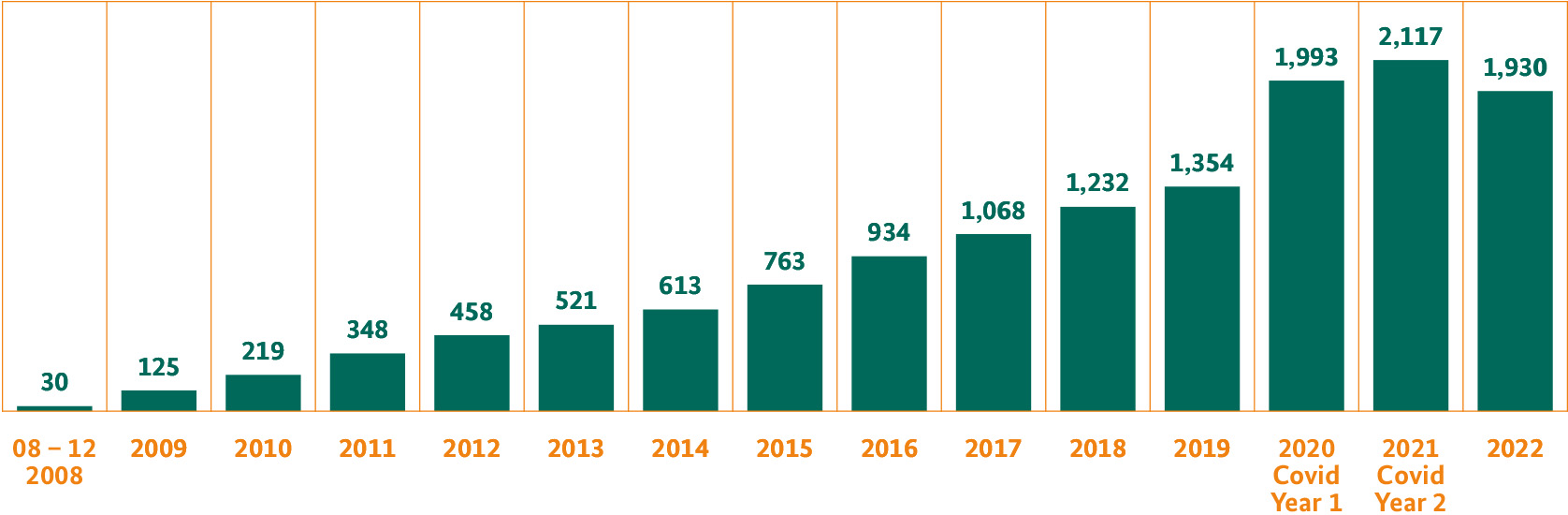
The German Clinical Trials Register (DRKS) is recognised by the World Health Organisation WHO as the primary register for Germany. It is responsible for the registration of all patient-oriented clinical trials carried out in Germany. The aim is to provide the public with a full, current overview of all clinical trials being carried out in Germany. The DRKS is an independent provider with public funding, which is operated by the BfArM. At the end of 2022, the register covered around 13,500 trials and recorded constant growth. Since 2016, the number of registrations has virtually doubled.
600
first applications
... for approval of a clinical trial processed by the BfArM in 2022.
12
GCP inspections
... (Good Clinical Practice) performed by the BfArM in 2021 and 2022.
Reporting side effects
Direct reports from Germany from patients
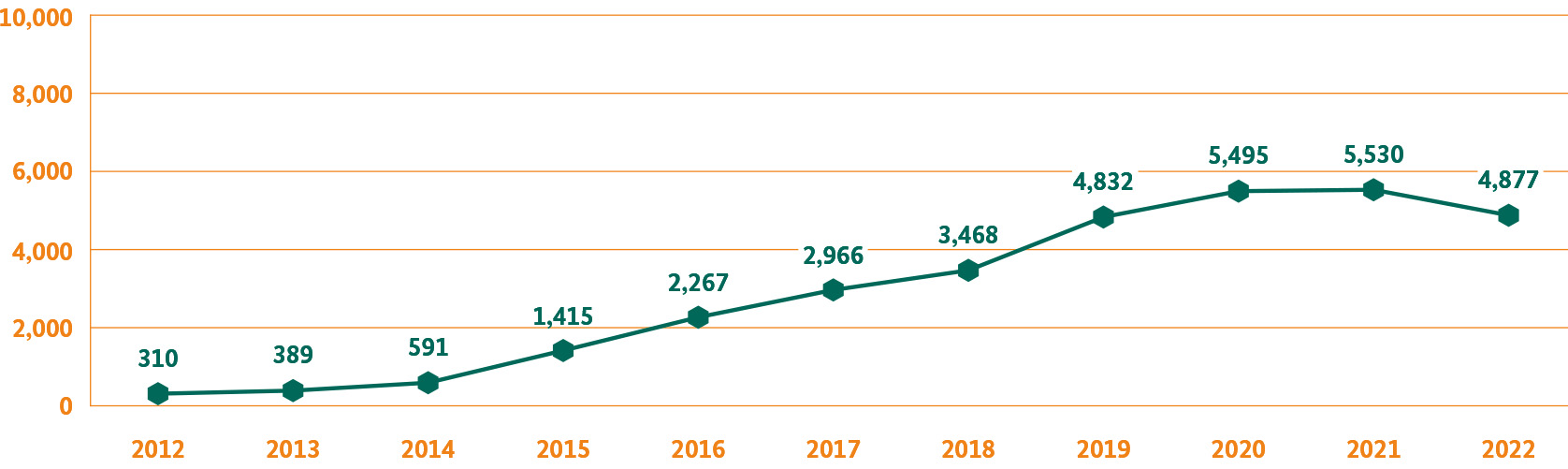
In conjunction with the PEI, the BfArM provides the online portal www.nebenwirkungen.bund.de, where adverse drug effects can be reported. Each report is quickly, directly and safely received from there by the experts from both authorities. Important: it is not only members of the medical profession who can report adverse events; patients and their family members or representatives may also do so. The BfArM has itself recorded continuous and significant growth in the number of direct reports. For example, reports by patients, their family members or representatives have grown 16-fold in the past ten years.
around 105.000
marketable medicines
... are under the BfArM‘s area of responsibility in Germany (as at July 2023).
288,845
suspected cases of side effects
... have been reported by Germany to the European Medicines Agency (EMA) in 2022. A total of 1,193,132 reports were received by the EMA.
970
reports of possible batch-related defects
... processed by the BfArM in 2022. This resulted in 75 recalls.
42
reports of suspected fraudulent cases
... were received via the international early warning system in 2022, largely discovered outside of the legal supply chain.
Risk reports for medical devices
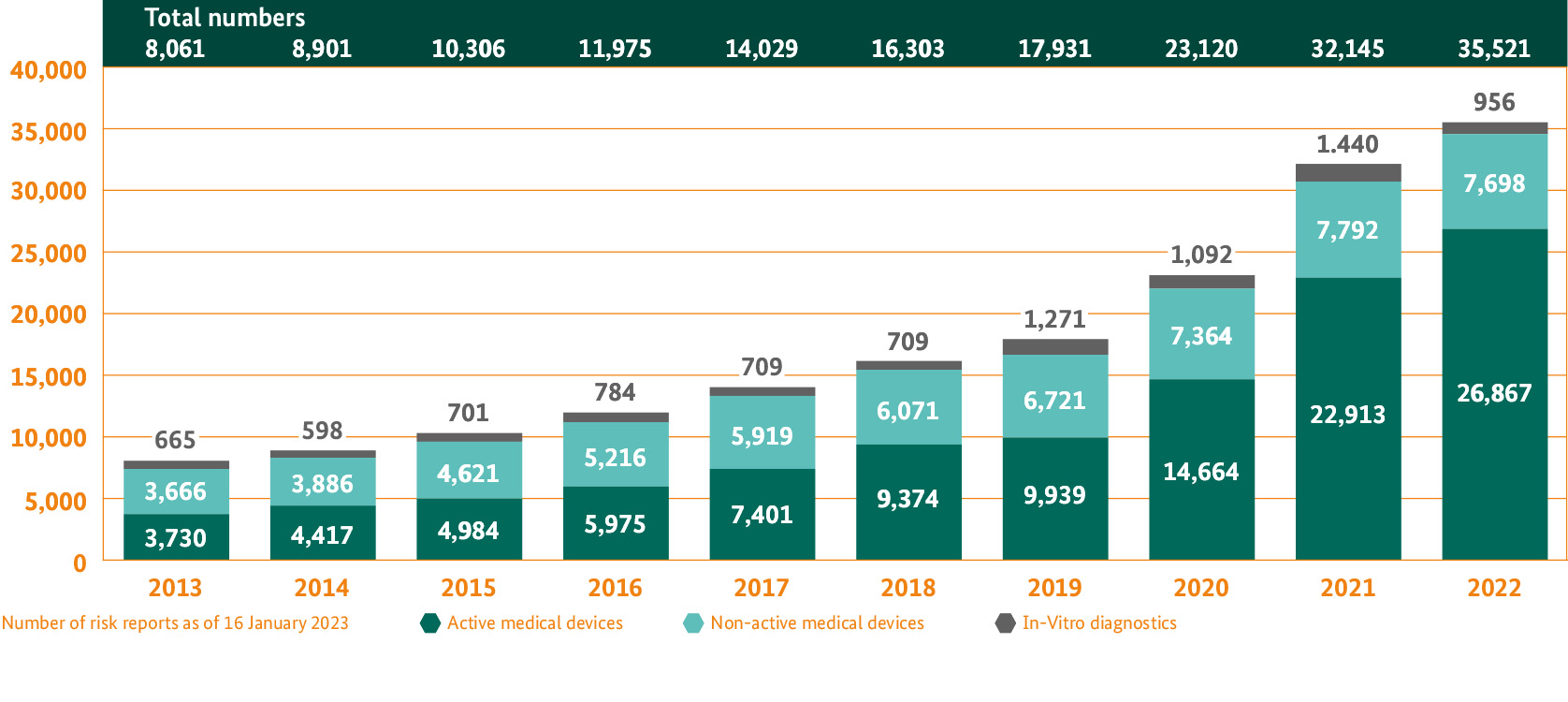
In the case of medical devices, the main task for the BfArM is the risk assessment of incidents. Manufacturers, authorised representatives and importers as well as users and other distributors are duty-bound to report incidents with medical devices to the BfArM in accordance with the provisions of the Medical Device Safety Plan Ordinance (MPSV).
Cannabis imports for medical and scientific purposes
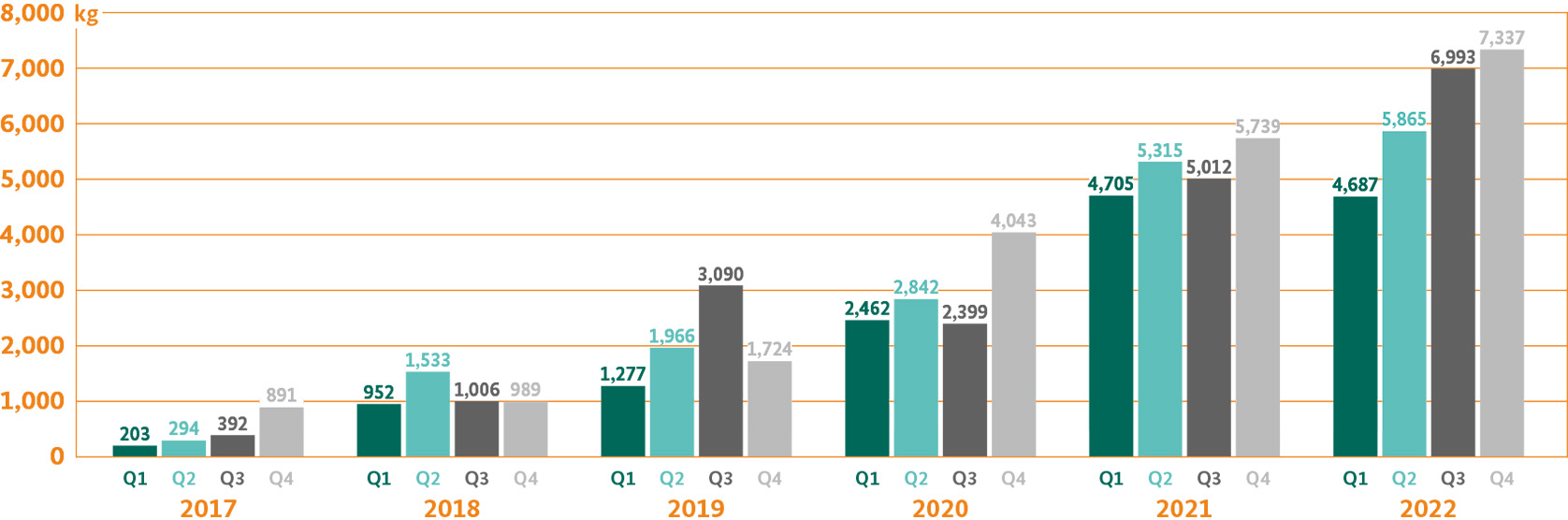
Medical cannabis may in principle be imported from any country, which carries out the cultivation of cannabis for medicinal purposes under state control in accordance with the Single Convention of 1961 on Narcotic Drugs and is able to offer cannabis in medicinal quality. The Federal Opium Agency at the BfArM issues the required permissions and licenses to companies interested in importing medicinal cannabis, however it does not have any centralised controlling role. It also has no influence on whether and to what extent the entitled companies are actually importing cannabis.
15.7 Mio.
anaesthetic prescriptions
... issued by the BfArM to doctors in 2022. The trend continues to rise slightly.
788
hectares
... cultivated for opium poppies (papaver somniferum) in 2022. First a reduction, after which the annual cultivated area reached a peak in 2021 with 1077 hectares.